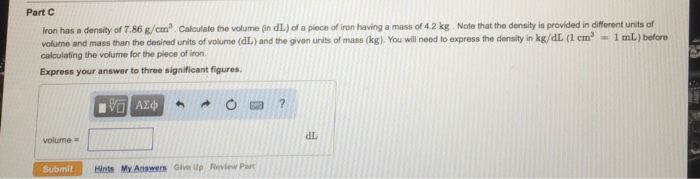
Iron has a density of 7.86 g cm3 – Iron, a transition metal renowned for its exceptional properties, possesses a density of 7.86 g cm3, making it a material of significant interest in various industries. This article delves into the physical and chemical characteristics of iron, exploring its density, applications, and implications for human health and the environment.
Iron’s density, a measure of its mass per unit volume, is a fundamental property that influences its behavior and suitability for specific applications. Understanding the density of iron provides insights into its strength, durability, and potential uses.
Properties of Iron
Iron is a metallic element with the symbol Fe (from Latin: ferrum) and atomic number 26. It is a transition metal in the first row of the d-block of the periodic table. Iron is a hard, malleable, and ductile metal.
It is the fourth most abundant element in the Earth’s crust, after oxygen, silicon, and aluminum.
Iron is an essential nutrient for plants and animals. It is used in the production of steel, cast iron, and wrought iron. It is also used in the manufacture of magnets, electrical equipment, and cookware.
Physical Properties
- Iron is a solid at room temperature.
- It has a melting point of 1,538 °C (2,800 °F) and a boiling point of 2,861 °C (5,182 °F).
- It is a good conductor of heat and electricity.
- It is magnetic.
- It is malleable and ductile.
Chemical Properties
- Iron is a reactive metal.
- It reacts with oxygen to form iron oxide.
- It reacts with water to form iron hydroxide.
- It reacts with acids to form iron salts.
Transition Metal
Iron is a transition metal because it has a partially filled d-orbital. This gives iron its magnetic properties.
Magnetic Properties
Iron is a ferromagnetic material. This means that it is attracted to magnets and can be magnetized.
Density of Iron: Iron Has A Density Of 7.86 G Cm3

Density is a fundamental property of matter that measures the mass of a substance per unit volume. It is an essential characteristic used to identify and differentiate materials.
The density of iron is 7.86 g/cm³, indicating that each cubic centimeter of iron has a mass of 7.86 grams. This value is experimentally determined by dividing the mass of a known volume of iron by the volume itself. Density is a valuable property for characterizing materials because it provides insights into their atomic packing, molecular structure, and intermolecular forces.
Comparison of Densities
The density of iron is relatively high compared to many other common materials. For example, the density of water is 1 g/cm³, wood is approximately 0.5 g/cm³, and aluminum is around 2.7 g/cm³. This higher density of iron contributes to its strength, durability, and resistance to deformation.
Applications of Iron

Iron is a versatile metal with a wide range of applications. Its unique properties, such as high strength, durability, and relatively low cost, make it a suitable material for various industries.
Iron is primarily used in the construction industry, where it is employed in the production of steel beams, rods, and other structural components. Steel is an alloy of iron and carbon, and its strength and durability make it ideal for use in buildings, bridges, and other large-scale structures.
Automotive Industry, Iron has a density of 7.86 g cm3
Iron is also a major component in the automotive industry. It is used in the production of car bodies, frames, and engines. The strength and durability of iron make it a suitable material for these applications, as it can withstand the stresses and vibrations associated with driving.
Machinery and Tools
Iron is widely used in the manufacturing of machinery and tools. Its strength and durability make it ideal for use in gears, bearings, and other components that are subjected to high levels of wear and tear. Iron is also used in the production of cutting tools, such as drills and saws, due to its hardness and ability to retain a sharp edge.
Advantages of Using Iron
- High strength and durability
- Relatively low cost
- Good electrical and thermal conductivity
- Versatile and can be easily alloyed with other metals
Disadvantages of Using Iron
- Susceptible to corrosion
- Can be brittle at low temperatures
- Relatively heavy
Health Implications of Iron

Iron is an essential mineral for the human body, playing a vital role in the production of red blood cells, which carry oxygen throughout the body. It is also involved in other important bodily functions, such as energy production, immune function, and cognitive development.
Maintaining healthy iron levels is crucial for overall well-being. Iron deficiency can lead to anemia, a condition characterized by a lack of red blood cells, resulting in fatigue, weakness, and shortness of breath. On the other hand, excessive iron intake can lead to iron overload, which can damage organs such as the liver and heart.
Iron Deficiency
Iron deficiency is a common nutritional problem, particularly among women, children, and individuals with certain medical conditions. Symptoms of iron deficiency include fatigue, weakness, shortness of breath, pale skin, and brittle nails. In severe cases, iron deficiency can lead to anemia, which can have serious health consequences.
To prevent iron deficiency, it is important to consume a diet rich in iron-containing foods, such as red meat, fish, beans, lentils, and leafy green vegetables. Iron supplements may also be necessary for individuals at risk of deficiency.
Iron Overload
Iron overload, also known as hemochromatosis, is a condition in which the body accumulates excess iron. This can occur due to genetic disorders, certain medical conditions, or excessive intake of iron supplements. Iron overload can damage organs such as the liver, heart, and pancreas, leading to serious health problems.
Treatment for iron overload typically involves removing excess iron from the body through phlebotomy, a procedure in which blood is drawn from a vein. In severe cases, liver transplantation may be necessary.
Maintaining Healthy Iron Levels
Maintaining healthy iron levels is essential for overall health. To achieve this, it is important to consume a balanced diet that includes iron-rich foods and to avoid excessive intake of iron supplements. Regular blood tests can help monitor iron levels and identify any potential problems.
Environmental Impact of Iron

Iron mining and production have significant environmental impacts, including air and water pollution, deforestation, and soil erosion. Mining operations release harmful gases and dust into the atmosphere, while wastewater from processing plants can contaminate water bodies.
To minimize the environmental footprint of iron production, measures such as using renewable energy sources, adopting energy-efficient technologies, and implementing waste reduction programs are being implemented. Recycling iron and its alloys is crucial as it reduces the need for mining and conserves natural resources.
Air Pollution
- Mining and processing operations release pollutants such as sulfur dioxide, nitrogen oxides, and particulate matter into the atmosphere.
- These pollutants contribute to air pollution, respiratory problems, and climate change.
Water Pollution
- Wastewater from iron processing plants contains heavy metals and other contaminants that can pollute water bodies.
- This pollution can harm aquatic life and affect drinking water quality.
Deforestation
- Mining operations often require clearing forests, leading to deforestation.
- Deforestation contributes to climate change, loss of biodiversity, and soil erosion.
Soil Erosion
- Mining activities can disrupt soil structure, leading to erosion.
- Soil erosion can result in loss of fertile land and increased sedimentation in water bodies.
Question & Answer Hub
What is the significance of iron’s density?
Iron’s density is a key factor in determining its strength, durability, and suitability for various applications. A higher density often indicates greater strength and resistance to deformation.
How is the density of iron determined?
The density of iron can be experimentally determined using methods such as the water displacement method or the Archimedes’ principle, which involves measuring the mass and volume of a sample.
What are some materials with similar densities to iron?
Materials with similar densities to iron include steel (7.85 g cm3), nickel (8.90 g cm3), and copper (8.96 g cm3).
